40 legal requirements for dispensing labels uk
Labelling standards - Pharmacy Forum UK 25/06/2010 · Labelling standards. 25, June 2010, 12:37 AM. Hi, A bit of background. Irish qualified pharmacist moved to UK. Working as relief for a large multiple for the last few months. One … Best practice guidance on the labelling and packaging of medicines to the Medicines and Healthcare products Regulatory Agency (MHRA) as any ... Labelling must contain all elements required by Regulation 257 and Schedule.
Labelling requirements for Prescription Only Medicines (POM) and ... Apr 27, 2022 ... In practice the medicine will usually require over-labelling by a licensed unit leaving a space for the individual's name, date of dispensing, ...

Legal requirements for dispensing labels uk
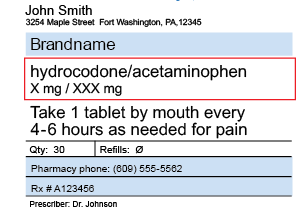
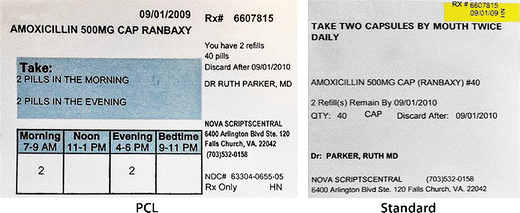
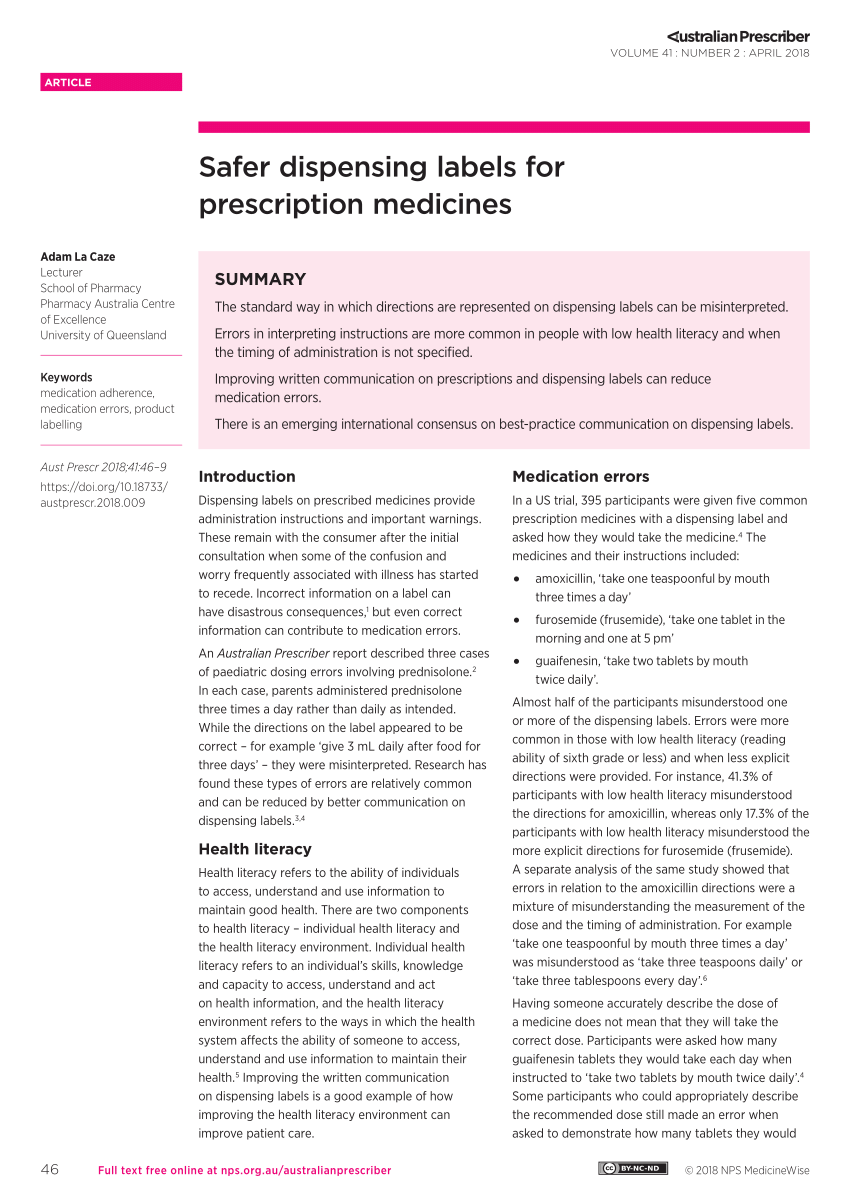
Pharmacy and Dispenser Label Solutions | Quality Label Printing UK Apr 7, 2020 ... Patient's prescription; Patients name and address; Dispensing date; Medicine name and details; Cautions and warnings. The label of prescribed ... Dispensing a prescription - PSNC Website 05/07/2013 · Dispensing a prescription. Published on: 5th July 2013 | Updated on: 1st April 2022. This section contains detailed information on dispensing all products other than for controlled … Optimising Dispensing Labels and Medicines Use This professional guidance is distinct from regulation, but supports pharmacist to meet applicable regulatory standards of conduct, ethics and performance or codes of ethics when undertaking …
Legal requirements for dispensing labels uk. United Kingdom - Labeling/Marking Requirements 12/09/2022 · UKCA markings must only be placed on a product by the manufacturer or an authorized representative. When affixing the UKCA marking, the manufacturer takes full … Medicines: packaging, labelling and patient information leaflets Dec 18, 2014 ... Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional ... Optimising Dispensing Labels and Medicines Use Decision pathway · Relevant clinical specialism and competency, knowledge and experience of pharmacist · Availability of information, such as: Patient ... Packaging & Labelling Requirements Guide | Handy Labels We’ve put together a list of the general food labelling requirements, based on the FSA labelling requirements. Name of food. The name of the food must be clearly presented and …
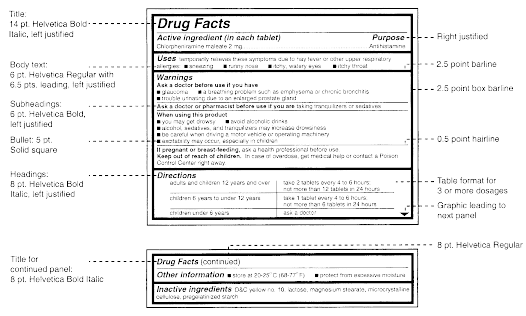
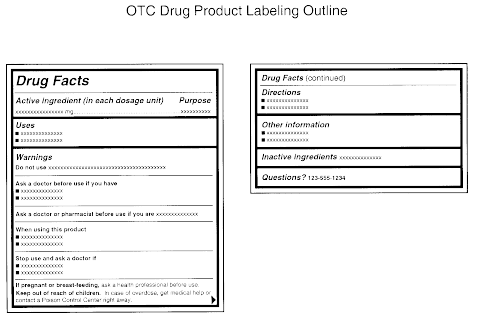
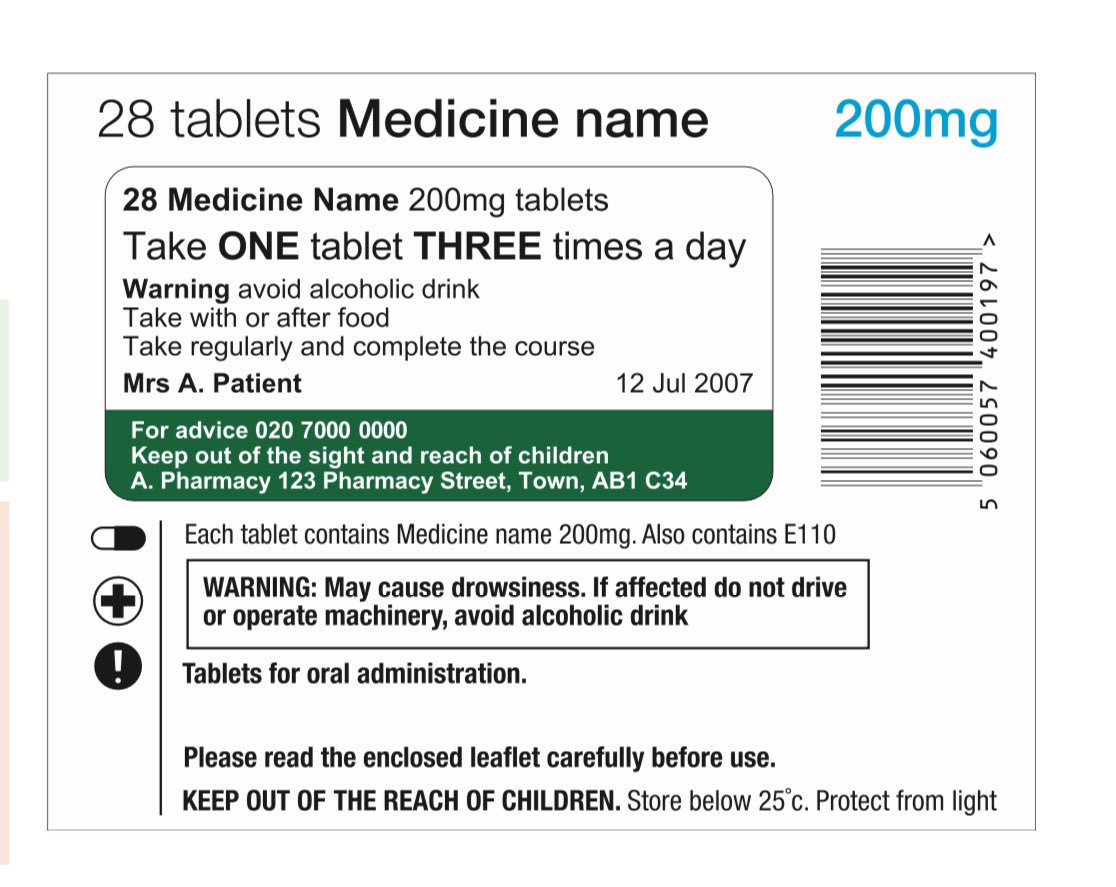
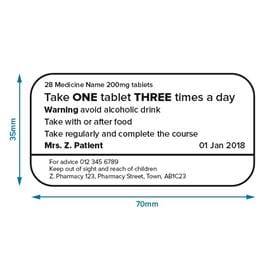
The Medicines (Labelling) Amendment Regulations 1992 Requirements are also imposed to the effect that the particulars on the label must be legible, comprehensible and in the English language. Particulars may appear in more than one … Best practice in the labelling and packaging of medicines - GOV.UK 29/12/2014 · As part of a move towards an increase in self-regulation of medicines labelling and packaging, this guidance has been developed to aid those responsible for the origination of … National standard for labelling dispensed medicines July 2021 The dispensed medicine label size used by many pharmacy dispensing systems is currently width. 80 mm × height 40 mm. 3.2 Label content. Legislative requirements ... Medicines: packaging, labelling and patient information … 18/12/2014 · Labels must be clear. Healthcare professionals and patients must easily be able to identify the medicine by the label. You should use the letters CD in an inverted triangle if your …
Supply of medicines | Medicines guidance - BNFC - NICE Labelling of prescribed medicines · name of the patient; · name and address of the supplying pharmacy; · date of dispensing; · name of the medicine; · directions for ... The Medicines (Labelling) Amendment Regulations 1992 “Standard labelling requirements for containers and packages for medicinal products for human use ... 4A.—(1) Except where paragraph (2) or (3) of this regulation ... Pharmacy dispensing models and displaying prices on … 11/11/2021 · clarify the current dispensing label requirements for monitored dosage systems ( mds) (medicine storage devices with compartments divided into days of the week and various … Best practice in the labelling and packaging of medicines - GOV.UK Dec 29, 2014 ... This guidance does not constitute a legal interpretation of the requirements on medicines labelling and packaging as set down within the Human ...
Product labelling: the law - GOV.UK Labels must not be misleading about things like: quantity or size the price what it’s made of how, where and when it was made what you say it can do the people or organisations that endorse it...
Controlled Drug prescription forms and validity - PSNC Website Sep 13, 2022 ... Identity checks: There is a legal requirement for pharmacists to establish whether a person collecting a Schedule 2 CD is the patient, the ...
Optimising Dispensing Labels and Medicines Use This professional guidance is distinct from regulation, but supports pharmacist to meet applicable regulatory standards of conduct, ethics and performance or codes of ethics when undertaking …
Dispensing a prescription - PSNC Website 05/07/2013 · Dispensing a prescription. Published on: 5th July 2013 | Updated on: 1st April 2022. This section contains detailed information on dispensing all products other than for controlled …
Pharmacy and Dispenser Label Solutions | Quality Label Printing UK Apr 7, 2020 ... Patient's prescription; Patients name and address; Dispensing date; Medicine name and details; Cautions and warnings. The label of prescribed ...
























Post a Comment for "40 legal requirements for dispensing labels uk"